Einführung in die Geschichte und Philosophie der Chemie
Kooperationsveranstaltung der Fakultäten für Chemie und für Geistes- und Sozialwissenschaften der Universität Karlsruhe (TH)
4. Vorlesung:Chemische Theorienbildung im 19. Jahrhundert |
Copyright Ó 2000 by Joachim Schummer
1.1 Korpuskulartheorien im 17. und 18. Jhd.
1.2 Affinitäten und Verbindungsgewichte
1.2.1 Relative Affinitätsstärken
1.2.2 Relative Verbindungsgewichte
1.3 Daltons Atomismus
1.4 Schwierigkeiten und Ergänzungen bei der Entwicklung des Systems von Äquivalentgewichten
1.5 Physikalischer Atomismus, Chemischer Atomismus und anti-Atomismus
2.1 Das Grundproblem: Klassifikation der organischen Verbindungen
2.2 Elektrischer Dualismus und die Radikaltheorie von Berzelius
2.3 Die Typentheorie von Laurent und Gerhardt
2.5. Die chemische Strukturtheorie von Kekulé
2.6. Stereochemie
|
|
|
| Daniel Sennert (1572-1637)
Pierre Gassendi (1592-1655) René Descartes (1596-1650) Robert Boyle (1627-91) Isaac Newton (1643-1727) François Etienne Geoffroy (1672-1731) Ernst Gottfried Fischer (1754-1831) Jeremias Benjamin Richter (1762-1807) John Dalton (1766-1844) Amedeo Avogadro (1776-1856) Joseph Louis Gay-Lussac (1778-1850) Jens Jakob Berzelius (1779-1848) William Prout (1785-1850) Pierre Louis Dulong (1785-1838) Alexis Thérèse Petit (1791-1820) |
Friedrich Wöhler (1800-82)
Jean Baptiste Dumas (1800-84) Justus von Liebig (1803-73) Auguste Laurent (1808-53) Charles Frédéric Gerhardt (1816-56) August Wilhelm von Hofmann (1818-92) Louis Pasteur (1822-1895) Alexander William Williamson (1824-1909) Stanislao Cannizzaro (1826-1910) Alexander Butlerow (1828-86) Friedrich August Kekulé (1829-96) Johannes Wislicenus (1835-1902) Joseph Achille Le Bel (1847-1930) Jacobus Henricus van’t Hoff (1852-1911) |

B + C ® BC qB,C = mB/mC
mD = qA,D mA = qA,D
qA,C 1000g
mH + mO ® mWassermH := mH
mO = cOmO
mWasser = cWasser mWasser = cWasser (mH + cO mO)
2 Vol. Wasserstoff + 1 Vol. Sauerstoff ® 2 Vol. Wasser2 Atome Wasserstoff + 1 Atom Sauerstoff ® 2 Atome Wasser (???)
|
|
|
|
|
| Berzelius (Schweden) |
|
|
|
| Liebig (Deutschland) |
|
|
|
| Dumas (Frankreich) |
|
|
|
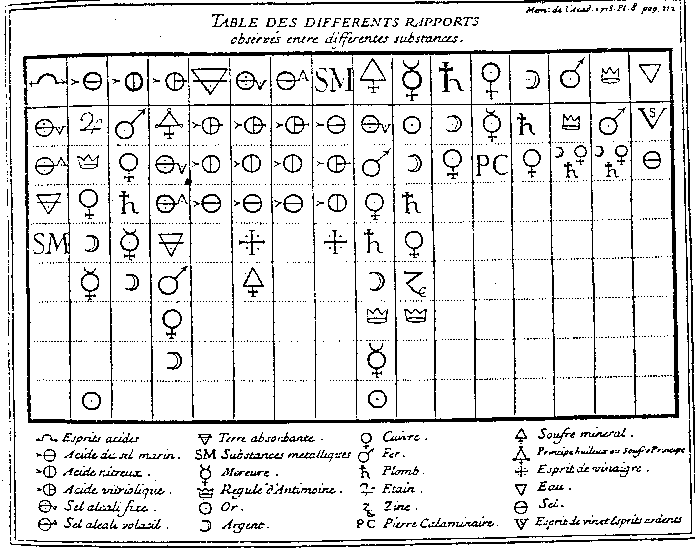
Erklärung der Lavoisierschen Stoffsystematik
M+ + O- ® (MO)- {z.B. CuO}Annahmen:X+ + O- ® (XO)- {z.B. SO3}
(MO)- + (XO)+ ® [(MO)(XO)] {z.B. CuSO4}
Schwefelsäure: [SO3 + H2O]Essigsäure(anhydrid): [(C4H6)O3 + H2O]
z.B. für Ethanol:Dumas: (C2H4)(H2O) (Wasseraddition an Ethen)
Berzelius: (C2H5O)(H) (Ethanolatbildung)
Liebig: (C2H3O)(3H)] (Oxidation zu Essigsäureanhydrid)
Essigsäure + Chlor ® Perchloressigsäure(CH3-COOH) ® (CCl3-COOH)
Experimentelle Substitution liefert Serien von chemisch ähnlichen StoffenCH4 CH3Cl CH2Cl2 CHCl3
C2H4 C2H5Br C2H4Br2 C2H3Br3
Formale Substitution liefert Serien von chemisch unähnlichen StoffenCH4 CH3Cl CH2O CHO2Na
Formaler Substitution der Kohlenwasserstoffgruppen liefert Serien von chemisch ähnlichen StoffenCH4O C2H6O C3H8O C4H10O etc. (+CH2)
CH2O2 C2H4O2 C3H6O2 C4H8O2 etc. (+CH2)
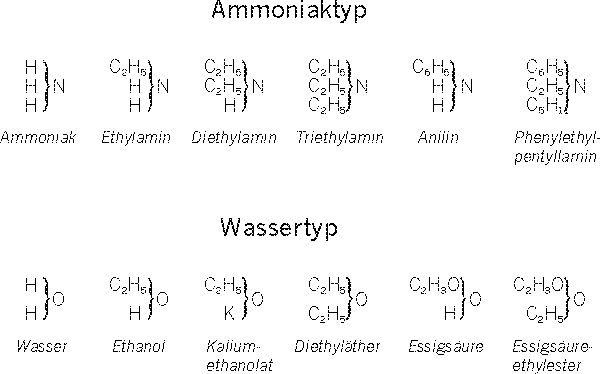

'Wurstformeln' für Methan und Methylchlorid
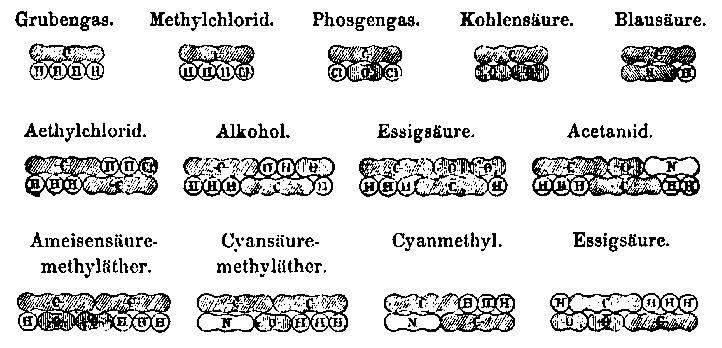
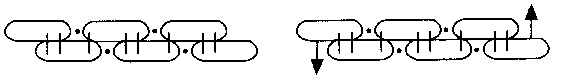
Kekulés ‘Wurstformeln’ für offene (links) und geschlossene
(rechts) 6-gliedrige Kohlenstoffketten mit alternierenden Einfach- und
Doppelverknüpfungen; das rechte Bild ist Kekulés ursprüngliche
Konstitutionsformel für Benzol
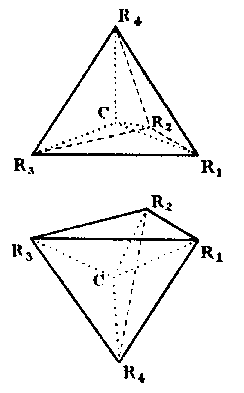
1. Assymmetrische Kohlenstoffatome
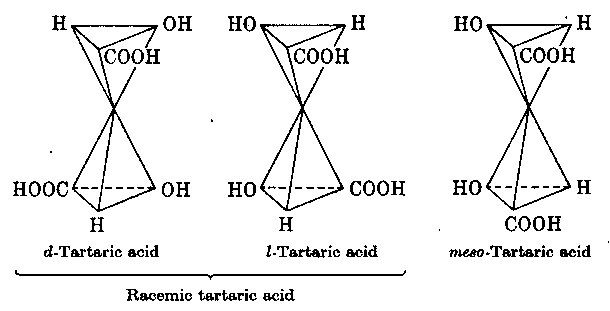
2. Strukturelle Differenzierung der 3 Weinsäuren
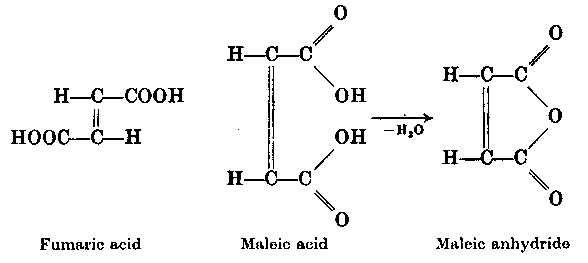
3. Strukturelle Deutung der Isomerie von Fumarsäure und Maleinsäure
(in heutiger Formelschreibweise)
Bloch, Ernst: 'Die antike Atomistik in der neueren Geschichte der Chemie', Isis, 1 (1913), 377-415.
Brocke, W.H. (Hg.): The Atomic Debates. Brodie and the Rejection of the Atomic Theory, Leicester: Univ. of Leicester Press, 1967.
Brooke, J. H.: 'Avogadro's Hypothesis and Its Fate: A Case-Study in the Failure of Case-Studies', History of Science, 19 (1981), 235-273.
Buchdahl, G.: 'Sources of Scepticism in Atomic Theory', The British Journal for the Philosophy of Science, 10 (1959), 120-134.
Cardwell, D. S. L. (Hg.): John Dalton & the Progress of Science, Manchester: Manchester Univ. Press; New York: Barnes & Noble, 1968.
Cole, Th. Jr.: 'Dalton, Mixed Gases, and the Origin of the Chemical Theory', Ambix, 25 (1978), 117-130.
Fisher, N. W.: 'The Nature of the Chemical Atom', History of Science, 11 (1973), 53-61.
Fleck, G. M.: 'Atomism in Late Nineteenth-Century Physical Chemistry', Journal of the History of Ideas, 24 (1963), 106-114.
Fleming, R. S.: John Dalton's Development of a Quantified Chemistry: A Reconstruction of the Genesis of Chemical Atomism. Univ. Cambridge (UK), 1981. (PhD Thesis).
Fleming, R. S.: 'Newton, Gases, and Daltonian Chemistry: The Foundations of Combination in Definite Proportion', Annals of Science, 31 (1974), 561-574.
Fuji, K.: 'The Berthollet-Proust Controversy and Dalton's Chemical Atomic Theory, 1800-1820', The British Journal for the History of Science, 19 (1986), 177-200.
Görs, B.: Chemischer Atomismus. Anwendung, Veränderung, Alternativen im deutschsprachigen Raum in der zweiten Hälfte des 19. Jahrhunderts, ERS-Verlag, Berlin, 1999.
Greenaway, F.: John Dalton and the Atom. London: Heinemann; Ithaca: Cornell Univ. Press, 1966.
Hall, M. B.: 'The Background of Dalton's Atomic Theory', Chemistry in Britain, 2 (1966), 341-345.
Kedrov, B. M.: 'Dalton's Atomic Theory and its Philosophical Significance', Philosophy and Phenomenological Research, 9 (1949), 644-662.
Knight, D. M.: Atoms and Elements. A Study of Theories of Matter in England in the Nineteenth Century. London, 1967.
Meinel, Ch.: 'Early 17th-Century Atomism: Theory, Epistemology, and the Insufficiency of Experiment', Isis, 79 (1988), 68-103.
Meldrum, A. N.: 'The Development of the Atomic Theory. (1) Berthollet's Doctrine of Variable Proportions', Memoirs and Proceedings of the Manchester Literary and Philosophical Society, 54(7), 1910.
Meldrum, A. N.: 'The Development of the Atomic Theory. (2) The Various Accounts of the Origin of Dalton's Theory', Memoirs and Proceedings of the Manchester Literary and Philosophical Society, 55(3), 1910-11.
Meldrum, A. N.: 'The Development of the Atomic Theory. (3) Newton's Theory and Its Influence in the Eighteenth Century', Memoirs and Proceedings of the Manchester Literary and Philosophical Society, 55(4), 1910-11.
Nash, L. K.: 'The Origin of Dalton's Chemical Atomic Theory', Isis, 47 (1956), 101-116.
Partington, J. R.: 'The Origins of the Atomic Theory', Annals of Science, 4 (1939), 245-282.
Partington, J. R.: 'Seventeenth-Century Chemistry, the Phlogiston Theory and Dalton's Atomic Theory', Nature, 174 (1954), 291-293.
Petrel, J.: La negation de l'atome dans la chimie du XIXe siècle: Cas de Jean-Baptiste Dumas (Cahiers d'histoire et de philosophie des sciences, 13). Paris: Centre National de la Recherche Scientifique, 1979.
Rocke, A. J.: 'Atoms and Equivalents: The Early Development of the Chemical Atomic Theory', The British Journal for the Philosophy of Science, 9 (1978), 225-263.
Rocke, A. J.: 'Gay-Lussac and Dumas: Adherents of the Avogadro-Ampère Hypothesis?', Isis, 69 (1978), 595-600.
Rocke, A. J.: 'The Reception of Chemical Atomism in Germany', Isis, 70 (1979), 519-536.
Rocke, A. J.: Chemical Atomism in the 19th Century: From Dalton to Cannizzaro, Columbus: Ohio State Univ. Press, 1984.
Rogers, M. J. W.: Dalton and the Atomic Theory. Harmondsworth: Penguin, 1966.
Roth, E.: 'Atomic Weights: Problems, Past and Present', Interdisciplinary Science Review, 2 (1977), 75-85.
Scheidecker-Chevalier, M.: ‘L’hypothèse d’Avogadro (1811) et d’Ampère (1814): la distinction atome/molécule et la theorie de la combinaison chimique’, Revue D’Histoire des Sciences, 50 (1997) 159-194.
Schofield, R. E.: 'Atomism from Newton to Dalton', American Journal of Physics, 49 (1981), 211-216.
Schönbeck, Ch. (Hg.): Atomvorstellungen im 19. Jahrhundert. Paderborn u.a.: Schöningh, 1982.
Schonland, B.: The Atomists (1805-1933). Oxford: Clarendon Press, 1968.
Shinn, T.: 'Orthodoxy and Innovation in Science: The Atomist Controversy in French Chemistry', Minerva, 18 (1980), 539-555.
Sinclair, S. B.: 'J. J. Thomson and the Chemical Atom: From Ether Vortex to Atomic Decay', Ambix, 34 (1987), 89-116.
Tanaka, M.: 'Über Ursprünge skeptischer Auffassungen gegen Atomhypothesen der Chemie des neunzehnten Jahrhunderts. Ein Beitrag zur Geschichte der Atomistik', Japanese Studies in the History of Science, 5 (1966), 87-99.
Tanaka, M.: 'Chemical and Physical Models for Atomistic Notion - Its
Conceptual Development in Relation to the Evolution of the Concept of Chemical
Substance.
A Contribution to the History of Atomism (IV)', Japanese Studies
in the History of Science, 8 (1969), 125-143.
Thackray, A. W.: Atoms and Powers: An Essay on Newtonian Matter-Theory and the Development of Chemistry. Cambridge: Cambridge Univ. Press, 1970.
Thackray, A. W.: 'The Emergence of Dalton's Chemical Atomic Theory: 1801-1808', The British Journal for the Philosophy of Science, 3 (1966), 1-23.
Thackray, A. W.: 'The Origins of Dalton's Chemical Atomic Theory: Daltonian
Doubts Resolved', Isis, 57 (1966), 35-55.
Brooke, J. H.: 'Chlorine Substitution and the Future of Organic Chemistry', Studies in the History and Philosophy of Science, 4 (1973), 47-94.
Brooke, J. H.: 'Laurent, Gerhardt, and the Philosophy of Chemistry', Historical Studies in the Physical Sciences, 6 (1975), 405-429.
Brooke, J. H.: 'Methods and Methodology in the Development of Organic Chemistry', Ambix, 34 (1987), 147-155.
Brooke, J. H.: 'Organic Syntheses and the Unificaton of Chemistry', The British Journal for the History of Science, 5 (1971), 363-392.
Byers, T.: 'The Radical, Dualism, and Auguste Laurent', Synthesis, 3 (1975), 22-37.
Fisher, N. W.: 'Organic Classification Before Kekule', Ambix, 20 (1973), 209-233.
Fisher, N. W.: 'Kekule and Organic Classification', Ambix, 21 (1974), 29-52.
Gay, H.: 'Radicals and Types: A Critical Comparison of the Methodologies of Popper and Lakatos and Their Use in the Reconstruction of Some 19th Century Chemistry', Studies in History and Philosophy of Science, 7 (1976), 1-51.
Larder, D.: 'A Dialectical Consideration of Butlerov's Theory of Chemical Structure', Ambix, 18 (1971), 26-48.
Ramberg, P.: ‘Pragmatism, Belief, and Reduction: Stereoformulas and Atomic Models in Early Stereochemistry’, HYLE - An International Journal for the Philosophy of Chemistry, 6 (2000).
Rocke, A. J.: 'Kekulé, Bulterov, and the Historiography of the Theory of Chemical Structure', The British Journal for the History of Science, 14 (1981), 27-57.
Rocke, A. J.: 'Subatomic Speculations and the Origin of Structure Theory', Ambix, 30 (1983), 1-18.
Rocke, A. J.: 'Hypothesis and Experiment in the Early Development of Kekule's Benzene Theory', Annals of Science, 42 (1985), 355-381.
Rocke, A. J.: 'Kekulé's Benzene Theory and the Appraisal of Scientific Theories'. in: Donovan, A. u. a. (Hg.): Scrutinizing Science: Empirical Studies of Scientific Change. Dordrecht: Kluwer Academic, 1988, S. 145-161.
Weyer, J.: 'Hundert Jahre Stereochemie - Ein Rückblick auf die
wichtigsten Entwicklungsphasen', Angewandte Chemie, 86 (1974),
604-11.
Bensaude-Vincent, B.: 'Mendeleev's Periodic System of Chemical Elements', The British Journal for the History of Science, 19 (1986), 3-17.
Emsley, J.: 'The Development of the Periodic Table of the Chemical Elements', Interdisciplinary Science Reviews, 12 (1987), 23-32.
Greenaway, F.: 'A Pattern of Chemistry: Hundred Years of the Periodic Table', Chemistry in Britain, 5 (1969), 97-99.
Hardt, H. D.: 'Zur hundertjährigen Geschichte des periodischen Systems der chemischen Elemente', Naturwissenschaftliche Rundschau, 19 (1966), 313-316.
Kedrov, B. M.: 'On the Question of the Psychology of Scientific Creativity. On the Occasion of the Discovery by D. I. Mendeleev of the Periodic Law', Soviet Review, 8-2 (1967), 26-45.
Kedrov, B. M.: 'Centenaire de la découverte de la loi périodique par D. I. Mendéléev', Actes XIIe Congr. Int. Hist. Sci., 6, 1968 (pub. 1971), 121-128.
Levi, P. & R. Rosenthal: The Periodic Table. New York: Schocken, 1984.
Mazurs, E. G.: Graphic Representations of the Periodic System during One Hundred Years. Alabama: Univ. Alabama Press, 1974.
Petrianov-Sokolov, I. V.: Elementary Order: Mendeleev's Periodic System (Transl. from the Russian by Weinstein, J.). Moscow: Mir, 1985. (Revised from the 1976 Russian Edition)
Rabinowitsch, E. & E. Thilo: Periodisches System. Geschichte und Theorie. Stuttgart: Enke, 1930.
Rancke-Madsen, E.: 'The Periodic System of Chemical Elements: An Essay Review', Centaurus, 18 (1973), 76-80.
Rawson, D. C.: 'The Process of Discovery: Mendeleev and the Periodic Law', Annals of Science, 31 (1974), 181-204.
Sambursky, S.: 'Structure and Periodicity - Centenary of Mendeleev's Discovery', Proceedings of the Israel Academy of Sciences and Humanities, 4-1, (1970), 1-13.
Scerri, E. R.: ‘The Evolution of the Periodic System’, Scientific American, 279 (1998), no. 3, 56-61.
Seaborg, G. T.: 'The Periodic Table: Tortuous Path to Man-Made Elements', Chemical and Engineering News, 57 (16), 1979, 46-52.
Spronsen, J. W. v.: 'Fundamentering van het periodiek systeem der elementen', Scientiarum Historia, 10 (1968), 106-109.
Spronsen, J. W. v.: 'Hundert Jahre Periodensystem der chemischen Elemente', NTM, 6-1 (1969), 13-42.
Spronsen, J. W. v.: L'histoire de la découverte du système périodique des éléments chimiques et l'apport de Béguyer de Chancourtois (Conference donnée au Palais de la Découverte, D 103). Paris: Presses Universitaires de France, 1965.
Spronsen, J. W. v.: The Periodic System of Chemical Elements: A History of the First Hundred Years. Amsterdam: Elsevier, 1969.
Spronsen, J. W. v.: 'The Prehistory of the Periodic System of the Elements',
Journal
of Chemical Education, 36 (1959), 565-567.